

mean field theories and other approximations do not take into account correlation, preventing the possibility of predicting stability for the negative hydrogen ion. Finding Ionic Radius Since ions tend to exist in bonds, the ionic radius can be found via the ionic bond between two atoms. We conclude that the negative hydrogen ion is a special atomic system whose stability depends completely on the electron correlation. The ionic radius can easily be a little smaller or larger than the atomic radius, which is the radius a neutral atom of the element possesses. The key concept introduced by Chandrasekhar was to break the symmetry between the two electrons, which is a way to introduce implicitly the electron correlation. The atomic radius is defined as the distance between the nucleus centre and the outermost shell containing the valence electron. But it was Chandrasekhar who first introduced a clever wave function to describe the H− system which leads to a beautiful physical picture. This site offers comprehensive information for each element including: who, when & where up to 40 properties (chemical & physical) over 3,600 nuclides (isotopes) over 4,400 nuclide decay modes the element names in 10 different languages and more. By choosing elements from the periodic table, atoms can be selected for a side by side comparison and analysis. Ionic radius values range from 31 pm to over 200 pm.
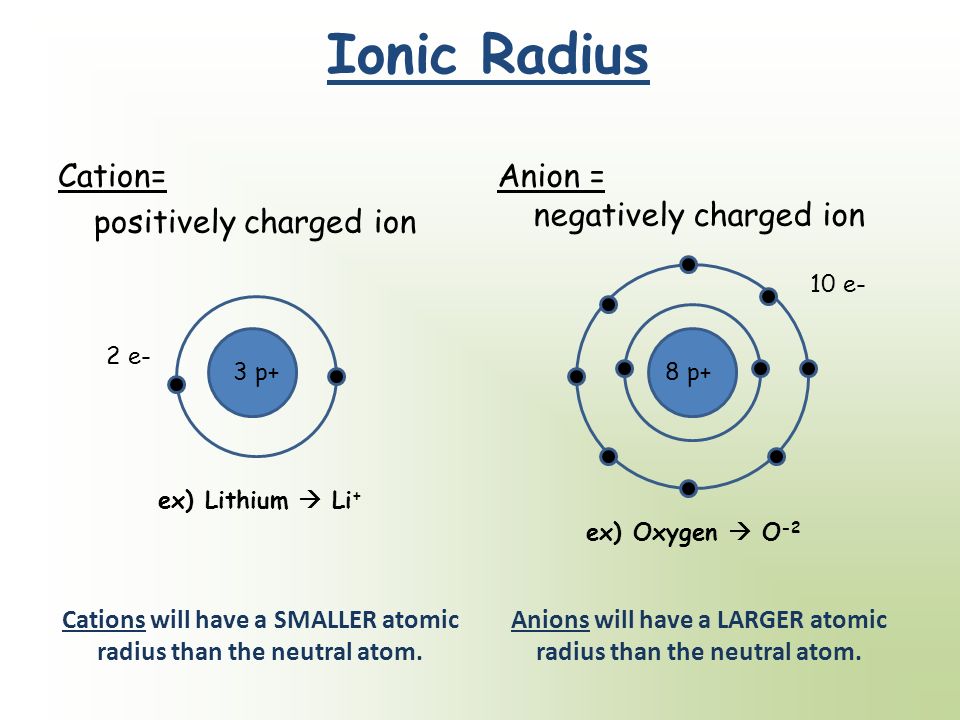
The ionic radius is the radius of a monatomic ion of an element within an ionic crystal or half the distance between two bonded gas atoms. In this simulation for the March 2016 issue, students can investigate the periodic trends of atomic radius, ionization energy, and ionic radius. While the atomic radius measures the size of a neutral atom, the ionic radius gauges the size of an electrically charged atom. Ions may be larger or smaller than the neutral atom, depending on the ions charge. In particular, it helps to drop the assumption that the electrons occupy the same spatial orbital and differ only according to their spin quantum number :Īs pointed out in the introduction Bethe and Hylleraas were the first authors to prove the stability of the negative hydrogen. Simulation: Periodic Trends: Ionization Energy, Atomic Radius & Ionic Radius. The neutral atoms are colored gray, cations red, and anions blue.

Electrostatic repulsion between electrons is the key to understanding $\ce$.


 0 kommentar(er)
0 kommentar(er)
